Disney Experience
Well-Known Member
For those curious on current covid treatment research:
Pfizer's Oral treatment: Double blinded (quadruple masked) Primary conpletion date Oct 16th this year :
 clinicaltrials.gov
clinicaltrials.gov

 www.medrxiv.org
www.medrxiv.org
Merck’s: Currently in Phase 3 trials
 www.nature.com
www.nature.com
/cloudfront-us-east-2.images.arcpublishing.com/reuters/QBG4W3IZINMVZJN5MRCGU676VE.jpg)
 www.reuters.com
www.reuters.com
There are a few other companies working on therapeutics. Hopefully the above ones have more success than previous ones, we should know soon.
Pfizer's Oral treatment: Double blinded (quadruple masked) Primary conpletion date Oct 16th this year :
ClinicalTrials.gov

An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19
The worldwide outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become an established global pandemic. Alongside vaccines, antiviral therapeutics are an important part of the healthcare response to counter the ongoing...
Merck’s: Currently in Phase 3 trials

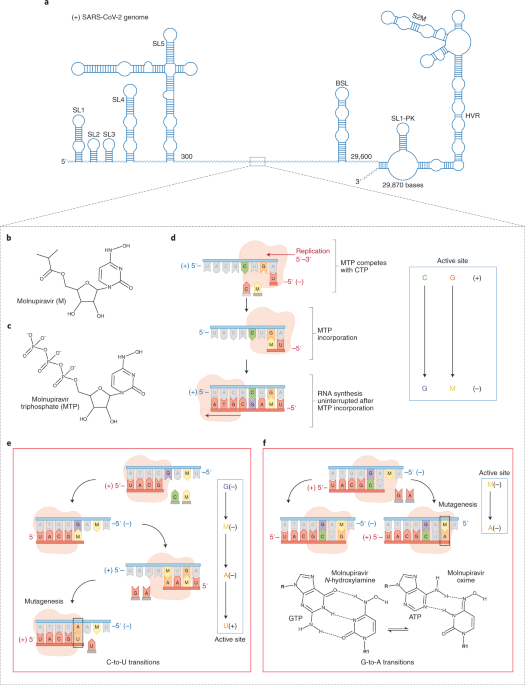
Molnupiravir: coding for catastrophe - Nature Structural & Molecular Biology
Molnupiravir, a wide-spectrum antiviral that is currently in phase 2/3 clinical trials for the treatment of COVID-19, is proposed to inhibit viral replication by a mechanism known as ‘lethal mutagenesis’. Two recently published studies reveal the biochemical and structural bases of how...
/cloudfront-us-east-2.images.arcpublishing.com/reuters/QBG4W3IZINMVZJN5MRCGU676VE.jpg)
Merck sees potential U.S. authorization for COVID-19 antiviral before year-end
Drugmaker Merck & Co Inc said on Monday it sees potential U.S. emergency use authorization for its experimental COVID-19 antiviral treatment, molnupiravir, before year-end.
There are a few other companies working on therapeutics. Hopefully the above ones have more success than previous ones, we should know soon.